甲基吡啶𬭩盐
外观
(重定向自甲基吡啶鎓阳离子)
| 甲基吡啶𬭩盐 | |
|---|---|
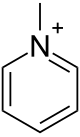
| |
| IUPAC名 1-Methylpyridinium | |
| 别名 | N-Methylpyridinium |
| 识别 | |
| CAS号 | 694-56-4 |
| PubChem | 13597 |
| ChemSpider | 13008 |
| SMILES |
|
| InChI |
|
| InChIKey | PQBAWAQIRZIWIV-UHFFFAOYAW |
| 性质 | |
| 化学式 | C6H8N+ |
| 摩尔质量 | 94.134 g/mol g·mol⁻¹ |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
甲基吡啶𬭩盐是一种吡啶的衍生物,可以通过吡啶的N-甲基化反应得到。在某些咖啡产品中可以找到它。 [1] 它不存在于未经烘焙的咖啡豆中,而是在烘焙过程中由其前体化学品葫芦巴碱形成的。 [1] 科学家正在研究它的潜在抗癌特性, [2] 特别是对结肠癌的作用。 [1]
离子液体
[编辑]甲基吡啶鎓离子的氯化物(N-甲基吡啶盐酸盐)在液态是一种离子液体。 几位作者在150 – 200°C(423 – 473 K)的温度范围内表征了甲基吡啶盐酸盐与氯化锌不同混合物的性能。 [3], [4], [5], [6]
参见
[编辑]参考资料
[编辑]- ^ 1.0 1.1 1.2 Highly Active Compound Found In Coffee May Prevent Colon Cancer. ScienceDaily. Oct 15, 2003 [2012]. (原始内容存档于2021-03-02).
- ^ Boettler, U; Volz, N; Pahlke, G; Teller, N; Kotyczka, C; Somoza, V; Stiebitz, H; Bytof, G; et al. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Molecular Nutrition & Food Research. 2011, 55 (5): 798–802. PMID 21448860. doi:10.1002/mnfr.201100115.
- ^ Simonis, L.; Coppe, C.; Glibert, J.; Claes, P. Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – I. Phase diagram and heats of mixing. Thermochimica Acta. 1986, 99: 223–232. doi:10.1016/0040-6031(86)85285-6.
- ^ Claes, P.; Simonis, L.; Glibert, J. Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – II. Specific mass, electrical conductivity and viscosity. Electrochimica Acta. 1986, 31 (12): 1525–1530. doi:10.1016/0013-4686(86)87071-2.
- ^ Claes, P. F.; Coppe, C. R.; Simonis, L. A.; Glibert, J. E. Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – III. Solubility of hydrogen chloride under atmospheric pressure and comparison with zinc chloride - N-ethylpyridinium bromide mixtures. Journal of Chemical and Engineering Data. 1987, 32 (1): 70–72. doi:10.1021/je00047a020.
- ^ Marković, R.; Minić, D. M. Conductometric and thermal studies of fused Zn (II) salts containing methyl substituted pyridinium cations. Materials Chemistry and Physics. 1997, 50 (1): 20–24. doi:10.1016/S0254-0584(97)80178-2.


