Β-連環蛋白
β-連環蛋白(β-catenin),也叫連環蛋白β-1(Catenin beta-1),是一種雙重功能蛋白質,參與細胞-細胞黏附和基因轉錄的調節和協調。在人類中,β-連環蛋白由CTNNB1基因編碼。[6][7]在果蠅中,同源蛋白稱為犰狳(armadillo)。β-連環蛋白是鈣黏素蛋白複合物的一個亞基,在Wnt信號通路中充當細胞內信號轉導子。[8][9][10]它是連環蛋白家族的成員,與γ-連環蛋白(也稱為斑珠蛋白)同源。 β-連環蛋白在許多組織中廣泛表達。在心肌中,β-連環蛋白定位於閏盤結構中的黏着連接處,這對於相鄰心肌細胞之間的電和機械耦合至關重要。
β-連環蛋白的突變和過表達與許多癌症有關,包括肝細胞癌、結直腸癌、肺癌、惡性乳腺腫瘤、卵巢癌和子宮內膜癌。[11]β-連環蛋白的定位和表達水平的改變與各種形式的心臟病有關,包括擴張型心肌病。β-連環蛋白由β-連環蛋白破壞複合物調節和破壞,特別是由腫瘤抑制APC基因編碼的腺瘤性結腸息肉(APC)蛋白調節和破壞。因此,APC基因的基因突變也與癌症密切相關,特別是由家族性腺瘤性息肉病(FAP)引起的結直腸癌。
發現
[編輯]β-連環蛋白最初是在1990年代初期作為哺乳動物細胞黏附複合物的一種成分被發現的:一種負責細胞質錨定鈣黏素的蛋白質。[12]但很快,人們意識到果蠅蛋白犰狳——參與介導Wingless/Wnt的形態發生效應——不僅在結構上與哺乳動物β-連環蛋白同源,而且在功能上也是同源。[13]因此,β-連環蛋白成為最早的兼職蛋白例子之一:一種執行不止一種完全不同的細胞功能的蛋白質。
結構
[編輯]蛋白質結構
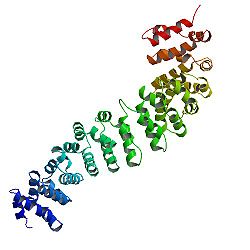
[編輯]β-連環蛋白的核心由幾個非常有特徵的重複序列組成,每個重複序列大約40個氨基酸長。稱為犰狳重複序列,所有這些元素摺疊在一起形成一個具有細長形狀的單一剛性蛋白質結構域,稱為犰狳(ARM)結構域。一個平均的犰狳重複由三個α螺旋組成。β-連環蛋白的第一個重複(靠近N端)與其他的略有不同——因為它有一個帶有扭結的細長螺旋,由螺旋1和2融合形成。[14]由於單個重複的形狀複雜,整個ARM域不是直杆:它具有輕微的曲率,因此形成了外(凸)和內(凹)表面。該內表面用作ARM結構域的各種相互作用夥伴的配體結合位點。

ARM域的N端和遠C端段本身在解中不採用任何結構。然而,這些本質上無序的區域在β-連環蛋白功能中起着至關重要的作用。N端天然無序區域包含一個保守的短線性基序,負責結合TrCP1(也稱為β-TrCP)E3泛素連接酶,但僅在它被磷酸化時。因此,β-連環蛋白的降解是由這個N末端片段介導的。另一方面,當C末端區域被招募到DNA上時,它是一個強大的反式活化因子。這個片段不是完全無序的:C末端延伸的一部分形成了一個穩定的螺旋,它與ARM結構域相結合,但也可能與單獨的結合夥伴結合。[15]這個小的結構元件(HelixC)覆蓋了ARM結構域的C末端,屏蔽了它的疏水殘基。β-連環蛋白不需要HelixC在細胞間黏附中發揮作用,但Wnt信號需要它:可能招募各種共活化因子,例如14-3-3zeta。[16]然而,它在一般轉錄複合物中的確切夥伴仍不完全清楚,它們可能涉及組織特異性參與者。[17]值得注意的是,如果人工融合到LEF1轉錄因子的DNA結合域,β-連環蛋白的C端片段可以模擬整個Wnt信號通路的作用。[18]
斑珠蛋白(也稱為γ-連環蛋白)具有與β-連環蛋白驚人相似的結構。不僅它們的ARM結構域在結構和配體結合能力方面彼此相似,而且N端β-TrCP結合基序在斑珠蛋白中也是保守的,這意味着共同的祖先和與β-連環蛋白的共同調控。[19]然而,當與DNA結合時,斑珠蛋白是一種非常弱的反式活化因子——這可能是由於它們的C末端序列的分歧造成的(斑珠蛋白似乎缺乏反式活化因子基序,因此抑制Wnt通路靶基因而不是活化它們)。[20]
綁定到犰狳域的合作夥伴
[編輯]
如上所示,β-連環蛋白的ARM結構域充當特定線性基序可能結合的平台。位於結構多樣的夥伴中,β-連環蛋白結合基序通常本身是無序的,並且通常在ARM結構域接合時採用剛性結構——如短線性基序所見。然而,β-連環蛋白相互作用的基序也具有許多獨特的特徵。首先,它們的長度可能達到甚至超過30個氨基酸的長度,並在過大的表面積上與ARM結構域接觸。這些基序的另一個不同尋常的特徵是它們經常高度磷酸化。此類Ser/Thr磷酸化事件極大地增強了許多β-連環蛋白相關基序與ARM結構域的結合。[21]
β-連環蛋白在複合物中的結構與轉錄反式活化夥伴TCF的連環蛋白結合域提供了有多少β-連環蛋白的結合夥伴可能形成相互作用的初始結構路線圖。[22]這種結構證明了TCF原本無序的N末端如何適應看似剛性的構象,結合基序跨越許多β-連環蛋白重複。定義了相對強的帶電相互作用「熱點」(預測並隨後驗證,對於β-連環蛋白/E-鈣黏蛋白相互作用是保守的),以及被認為在整體結合模式中重要的疏水區域和潛在的治療小分子抑制劑針對某些癌症形式。此外,以下研究證明了另一個獨特的特徵,即TCF的N末端與β-連環蛋白結合的可塑性。[23][24]
同樣,我們發現了熟悉的E-鈣黏蛋白,其細胞質尾部以相同的規範方式與ARM結構域接觸。[25]支架蛋白質軸蛋白(兩個密切相關的旁系同源物軸蛋白1和軸蛋白2)在其長而無序的中間部分包含類似的相互作用基序。[26]儘管一個軸蛋白分子僅包含一個β-連環蛋白募集基序,但其家族性腺瘤性息肉病(FAP)蛋白每個原體含有11個串聯排列的此類基序,因此能夠同時與多個β-連環蛋白分子相互作用。[27]由於ARM結構域的表面在任何給定時間通常只能容納一個肽基序,因此所有這些蛋白質都競爭相同的β-連環蛋白分子細胞池。這個比賽是了解Wnt信號通路如何工作的關鍵。
然而,ARM結構域β-連環蛋白上的這個「主要」結合位點絕不是唯一的。ARM結構域的第一個螺旋形成了一個額外的、特殊的蛋白質-蛋白質相互作用口袋:這可以容納在共活化因子BCL9(或密切相關的BCL9L)中發現的螺旋形成線性基序,一種參與Wnt信號傳導的重要蛋白質。[28]雖然精確的細節不太清楚,但當β-連環蛋白定位於黏附連接時,α-連環蛋白似乎使用了相同的位點。[29]因為這個口袋不同於ARM結構域的「主要」結合位點,α-連環蛋白和E-鈣黏蛋白之間或TCF1和BCL9之間分別沒有競爭。[30]另一方面,BCL9和BCL9L必須與α-連環蛋白競爭以獲取β-連環蛋白分子。[31]
功能
[編輯]通過磷酸化調節降解
[編輯]β-連環蛋白的細胞水平主要受其泛素化和蛋白酶體降解的控制。 E3泛素連接酶TrCP1(也稱為β-TrCP)可以通過無序N末端上的短線性基序將β-連環蛋白識別為其基質。然而,β-連環蛋白的這個基序(Asp-Ser-Gly-Ile-His-Ser)需要在兩個絲氨酸上被磷酸化才能結合β-TrCP。基序的磷酸化由糖原合酶激酶3α和β(GSK-3α和GSK-3β)進行。GSK-3是組成型活性酶,涉及幾個重要的調節過程。不過,有一個要求:GSK-3的基質需要在實際靶位點下游(C端)的四個氨基酸進行預磷酸化。因此,它的活性還需要「啟動激酶」。在β-連環蛋白的情況下,最重要的啟動激酶是酪蛋白激酶1。一旦「引發」了富含絲氨酸-蘇氨酸的基質,GSK-3就可以從C端到N端方向「穿過」它,連續磷酸化每4個絲氨酸或蘇氨酸殘基。該過程也將導致上述β-TrCP識別基序的雙重磷酸化。
β-連環蛋白破壞複合物
[編輯]GSK-3要成為基質上的高效激酶,預磷酸化是不夠的。還有一個額外的要求:與絲裂原活化蛋白激酶(MAPK)類似,基質需要通過高親和力對接基序與這種酶結合。β-連環蛋白不包含這樣的基序,但一種特殊的蛋白質包含:軸蛋白。更重要的是,它的GSK3對接基序與β-連環蛋白結合基序直接相鄰。[26]通過這種方式,軸蛋白充當了真正的支架蛋白質,將酶(GSK-3)與其基質(β-連環蛋白)結合在一起,在物理上非常接近。

但即使軸蛋白也不會單獨行動。通過其G蛋白信號調節因子(RGS)結構域的N端調節劑,它招募結腸腺瘤性息肉病(APC)蛋白。APC就像一棵巨大的「聖誕樹」:擁有眾多的β-連環蛋白結合基序(一個APC分子單獨擁有11個這樣的基序[27]),它可以收集儘可能多的β-連環蛋白分子。[32]APC可以同時與多個軸蛋白分子相互作用,因為它具有三個SAMP基序(Ser-Ala-Met-Pro)以結合軸蛋白中的RGS結構域。此外,軸蛋白還具有通過其C端DIX結構域寡聚化的潛力。結果是一個巨大的、多聚體的蛋白質組裝體,專門用於β-連環蛋白磷酸化。這種複合物通常稱為β-連環蛋白破壞複合物,儘管它與實際負責β-連環蛋白降解的蛋白酶體機制不同。[33]它僅標記β-連環蛋白分子以進行後續破壞。
Wnt信號與破壞調控
[編輯]在靜息細胞中,軸蛋白分子通過它們的C端DIX結構域相互寡聚化,DIX結構域具有兩個結合界面。因此,它們可以在細胞質內構建線性低聚物甚至聚合物。DIX結構域是獨特的:已知具有DIX結構域的唯一其他蛋白質是散亂蛋白和DIXDC1(果蠅的單個散亂蛋白對應於哺乳動物中的三個旁系同源基因Dvl1、Dvl2和Dvl3)。散亂蛋白與捲曲受體的細胞質區域及其PDZ和DEP結構域相關聯。當Wnt分子與捲曲受體結合時,它會引發一連串鮮為人知的事件,從而導致散亂蛋白的DIX結構域暴露,並為軸蛋白創建一個完美的結合位點。然後通過Dsh將軸蛋白從其寡聚組裝體(β-連環蛋白破壞複合物)中滴定出來。[34]一旦與受體複合物結合,軸蛋白將無法結合β-連環蛋白和GSK-3活性。重要的是,捲曲蛋白相關的LRP5和LRP6蛋白的細胞質片段包含GSK-3假基質序列(Pro-Pro-Pro-Ser-Pro-x-Ser),由酪蛋白激酶1適當地「引發」(預磷酸化),好像它是GSK-3的真正基質。這些錯誤的目標位點以競爭方式極大地抑制了GSK-3的活性。[35]通過這種方式,受體結合軸蛋白將消除介導β-連環蛋白的磷酸化。由於β-連環蛋白不再被標記為破壞,而是繼續產生,其濃度將增加。一旦β-連環蛋白水平升高到足以使細胞質中的所有結合位點飽和,它也會轉移到細胞核中。在與轉錄因子LEF1、TCF1、TCF2或TCF3結合後,β-連環蛋白會迫使它們脫離之前的夥伴:Groucho蛋白。與招募轉錄抑制子(例如組蛋白-離胺酸甲基轉移酶)的Groucho不同,β-連環蛋白將結合轉錄活化因子,開啟靶基因。
在細胞-細胞黏附中的作用
[編輯]
細胞-細胞黏附複合物對於複雜動物組織的形成至關重要。 β-連環蛋白是形成黏附連接的蛋白質複合體的一部分。[36]這些細胞-細胞黏附複合物對於上皮細胞層和屏障的產生和維持是必需的。作為複合物的組成部分,β-連環蛋白可以調節細胞生長和細胞間的黏附。它還可能負責傳遞接觸抑制信號,一旦上皮層完成,就會導致細胞停止分裂。[37]E-鈣黏蛋白-β-連環蛋白-α-連環蛋白複合物與肌動蛋白絲弱相關。黏附連接需要顯着的蛋白質動力學才能連接到肌動蛋白血球骨架,[36]從而實現機械力轉導。[38][39]
黏附連接的一個重要組成部分是鈣黏蛋白。鈣黏蛋白形成稱為黏附連接的細胞-細胞連接結構以及橋粒。鈣黏蛋白能夠通過其細胞外鈣黏蛋白重複結構域以Ca2+依賴性方式進行同源性相互作用;這可以將相鄰的上皮細胞保持在一起。在黏附連接處,鈣黏蛋白將β-連環蛋白分子募集到其細胞內區域。[需要解釋]反過來,β-連環蛋白與另一種高度動態的蛋白質α-連環蛋白結合,後者直接與肌動蛋白絲結合。[40]這是可能的,因為α-連環蛋白和鈣黏蛋白在不同的位點與β-連環蛋白結合。[41]因此,β-連環蛋白-α-連環蛋白複合物可以在鈣黏蛋白和肌動蛋白血球骨架之間形成物理橋樑。[42]鈣黏蛋白-連環蛋白複合物的組織還通過其成分的磷酸化和胞吞作用進行調節。[來源請求]
在發展中的作用
[編輯]β-連環蛋白在指導幾個發育過程中起着核心作用,因為它可以直接結合轉錄因子並受可擴散的細胞外物質Wnt的調節。它作用於早期胚胎以誘導整個身體區域以及發育後期的單個細胞。它還調節生理再生過程。
早期胚胎模式
[編輯]Wnt信號和β-連環蛋白依賴性基因表達在早期胚胎不同身體區域的形成過程中起關鍵作用。不表達這種蛋白質的實驗性改良胚胎將無法發育中胚層並啟動原腸胚形成。[43]在囊胚和原腸胚階段,Wnt以及骨塑型蛋白和纖維母細胞生長因子通路將誘導前後軸形成,調節原始條紋的精確位置(原腸胚形成和中胚層形成)以及神經形成過程(中樞神經系統發育)。[44]
在爪蟾卵母細胞中,β-連環蛋白最初同樣定位於卵子的所有區域,但它被 β-連環蛋白破壞複合物靶向泛素化和降解。卵子的受精導致外皮層旋轉,將捲曲蛋白和散亂蛋白簇移動到更靠近赤道區域的位置。在Wnt信號通路的影響下,β-連環蛋白會在繼承這部分細胞質的細胞中局部富集。它最終會轉移到細胞核以結合TCF3以活化幾個誘導背側細胞特徵的基因。[45]這種信號傳導導致了一個被稱為灰色新月體的細胞區域,它是胚胎發育的經典組織者。如果通過手術從胚胎中移除該區域,則根本不會發生原腸胚形成。 β-連環蛋白在胚孔唇的誘導中也起着至關重要的作用,這反過來又會引發原腸胚形成。[46]通過注射反義mRNA抑制GSK-3轉譯可能會導致形成第二個胚孔和多餘的體軸。 β-連環蛋白的過表達也會產生類似的效果。[47]
不對稱細胞分裂
[編輯]β-連環蛋白還涉及通過模式生物秀麗隱杆線蟲中的不對稱細胞分裂調節細胞命運。與爪蟾卵母細胞類似,這本質上是母細胞細胞質中散亂蛋白、捲曲受體、軸蛋白和APC分佈不均的結果。[48]
幹細胞更新
[編輯]Wnt信號傳導和某些細胞類型中β-連環蛋白水平升高的最重要結果之一是維持多潛能性。[44]在其他細胞類型和發育階段,β-連環蛋白可能促進分化,尤其是向中胚層細胞譜系分化。
β-連環蛋白還在胚胎發育的後期充當形態發生素。與TGF-β一起,β-連環蛋白的一個重要作用是誘導上皮細胞的形態發生變化。它促使它們放棄緊密的黏附,並呈現出更具流動性和鬆散關聯的間充質表型。在此過程中,上皮細胞會失去E-鈣黏素、緊密連接蛋白1(ZO-1)和細胞角蛋白等蛋白質的表達。同時,它們開啟波形蛋白、α平滑肌肌動蛋白(ACTA2)和纖維母細胞特異性蛋白1 (FSP1) 的表達。它們還產生細胞外基質成分,例如I型膠原蛋白和纖連蛋白。Wnt通路的異常活化與纖維化和癌症等病理過程有關。[49]在心肌發育中,β-連環蛋白發揮雙相作用。最初,Wnt/β-連環蛋白的活化對於間充質細胞進入心臟譜系至關重要。然而,在發育的後期階段,β-連環蛋白的下調是必需的。[50][51][43]
參與心臟生理學
[編輯]在心肌中,β-連環蛋白與N-鈣黏蛋白在閏盤結構內的黏附連接處形成複合物,負責相鄰心肌細胞的電氣和機械耦合。對成年大鼠心室心肌細胞模型的研究表明,β-連環蛋白的出現和分佈在培養中這些細胞的再分化過程中受到時空調節。具體來說,β-連環蛋白是具有N-鈣黏蛋白和α-連環蛋白的獨特複合物的一部分,在心肌細胞分離後的早期階段,它在黏附連接處豐富,以重建細胞-細胞接觸。[52]已經表明,β-連環蛋白在閏盤內的黏附連接處的心肌細胞中與艾默里蛋白形成複合物。這種相互作用取決於β-連環蛋白上GSK-3β磷酸化位點的存在。敲除埃默里蛋白顯着改變了β-連環蛋白定位和整體閏盤結構,類似於擴張型心肌病表型。[53]
在心臟病的動物模型中,β-連環蛋白的功能已被揭示。在主動脈瓣狹窄和左心室肥大的豚鼠模型中,儘管β-連環蛋白的整體細胞豐度沒有變化,但β-連環蛋白被證明可以改變從閏盤到胞質溶膠的亞細胞定位。紐蛋白顯示出類似的變化情況。N-鈣黏蛋白沒有變化,在沒有β-連環蛋白的情況下,閏盤處的斑珠蛋白沒有代償性上調。[54]在心肌病和心力衰竭的倉鼠模型中,細胞-細胞黏附不規則且雜亂無章,黏附連接/閏盤和β-連環蛋白的細胞核池的表達水平降低。[55]這些數據表明,β-連環蛋白的丟失可能在與心肌肥大和心力衰竭相關的患病閏盤中起作用。在心肌梗塞的大鼠模型中,非磷酸化的組成型活性β-連環蛋白的腺病毒基因轉移降低了心肌梗塞的大小,活化了細胞周期,並減少了心肌細胞和心肌纖維母細胞的凋亡量。這一發現與促存活蛋白、生存素和Bcl-2以及血管內皮生長因子的表達增強相一致,同時促進了心臟纖維母細胞向肌纖維母細胞的分化。這些發現表明,β-連環蛋白可以促進心肌梗塞後的再生和癒合過程。[56]在自發性高血壓心力衰竭大鼠模型中,研究人員檢測到β-連環蛋白從閏盤/肌膜穿梭到細胞核,這可以通過膜蛋白部分中β-連環蛋白表達的減少和核部分的增加來證明。此外,他們發現GSK-3β和β-連環蛋白之間的關聯減弱,這可能表明蛋白質穩定性發生了改變。總體而言,結果表明增強的β-連環蛋白核定位可能在心臟肥大的進展中很重要。[57]
關於β-連環蛋白在心臟肥大中的機製作用,轉基因小鼠研究表明,關於β-連環蛋白上調是有益還是有害的結果有些矛盾。[58][59][60]最近一項使用條件性敲除小鼠的研究,要麼完全缺乏β-連環蛋白,要麼在心肌細胞中表達不可降解形式的β-連環蛋白,從而調和了這些差異的潛在原因。這似乎對β-連環蛋白在心肌中的亞細胞定位有嚴格的控制。缺乏β-連環蛋白的小鼠在左心室心肌中沒有明顯的表型;然而,攜帶穩定形式的β-連環蛋白的小鼠發展為擴張型心肌病,這表明通過蛋白質降解機制對β-連環蛋白的時間調節對於心臟細胞中β-連環蛋白的正常功能至關重要。[61]在一個小鼠模型中,敲除與致心律不整性右心室心肌病有關的橋粒蛋白、斑珠蛋白,β-連環蛋白的穩定性也得到了增強,可能是為了彌補其斑珠蛋白同系物的損失。這些變化與Akt活化和GSK-3β抑制相協調,再次表明β-連環蛋白的異常穩定可能與心肌病的發展有關。[62]對斑珠蛋白和β-連環蛋白進行雙重敲除的進一步研究表明,雙重敲除會導致心肌病、纖維化和心律不整,從而導致心源性猝死。閏盤結構嚴重受損,連接蛋白43駐留間隙連接明顯減少。心電圖測量在雙轉基因動物中捕獲了自發性致死性室性心律不整,這表明兩種連環蛋白——β-連環蛋白和斑珠蛋白對於心肌細胞中的機械電耦合至關重要且必不可少。[63]
臨床意義
[編輯]在抑鬱症中的作用
[編輯]根據西奈山伊坎醫學院於2014年11月12日發表在《自然》雜誌上的一項研究,一個特定個體的大腦是否能夠有效地應對壓力以及他們對抑鬱症的易感性,取決於每個人大腦中的β-連環蛋白。[64]較高的β-連環蛋白信號會增加行為靈活性,而有缺陷的β-連環蛋白信號會導致抑鬱和壓力管理減少。[64]
在心臟病中的作用
[編輯]β-連環蛋白中改變的表達譜與人類擴張型心肌病有關。通常在擴張型心肌病患者中觀察β-連環蛋白表達上調。[65]在一項特定的研究中,終末期擴張型心肌病患者的雌激素受體α(ER-α)mRNA和蛋白質水平幾乎翻了一番,並且ER-α/β-連環蛋白相互作用,存在於對照、非患病人類的閏盤上心臟丟失,表明閏盤處這種相互作用的喪失可能在心力衰竭的進展中起作用。[66]與BCL9和PYGO蛋白一起,β-連環蛋白協調聽覺發育的不同方面,模型生物(如小鼠和斑馬魚)中Bcl9或Pygo的突變導致與人類先天性心臟病非常相似的表型。[67]
參與癌症
[編輯]
β-連環蛋白是一種原癌基因。該基因的突變常見於多種癌症中:原發性肝細胞癌、大腸癌、卵巢癌、乳癌、肺癌和膠質母細胞瘤。據估計,從所有癌症中測序的所有組織樣本中約有10%顯示CTNNB1基因突變。[68]大多數這些突變聚集在β-連環蛋白N末端片段的一小塊區域:β-TrCP結合基序。該基序的功能喪失突變基本上使β-連環蛋白的泛素化和降解成為不可能。它將導致β-連環蛋白在沒有任何外部刺激的情況下易位至細胞核並持續驅動其靶基因的轉錄。在基底細胞癌、[69]頭頸部鱗狀細胞癌、前列腺癌、[70]毛母質瘤[71]和髓母細胞瘤[72]中也注意到細胞核β-連環蛋白水平升高。這些觀察結果可能或不暗示β-連環蛋白基因的突變:其他Wnt通路成分也可能有缺陷。
在APC的β-連環蛋白募集基序中也經常看到類似的突變。APC的遺傳性功能喪失突變導致稱為家族性腺瘤性息肉病的病症。受影響的個體在其大腸中長出數百個息肉。這些息肉多數本質上是良性的,但隨着時間的推移,它們有可能轉變為致命的癌症。大腸癌中APC的體細胞突變也並不少見。[73]β-連環蛋白和APC是參與大腸癌發展的關鍵基因(連同其他基因,如K-Ras和SMAD4)。β-連環蛋白將受影響細胞的先前上皮表型改變為侵襲性間充質樣類型的潛力極大地促進了轉移的形成。
作為治療靶點
[編輯]由於其參與癌症發展,β-連環蛋白的抑制作用繼續受到極大關注。但是,由於其廣泛且相對平坦的表面,在其犰狳結構域上定位結合位點並不是最簡單的任務。然而,與該表面的較小「熱點」結合就足夠有效抑制它了。這樣一來,源自LEF1中發現的天然β-連環蛋白結合基序的「釘合」螺旋肽足以完全抑制β-連環蛋白依賴性轉錄。最近,幾種小分子化合物被研發來靶向ARM結構域的相同的高正電荷區域(CGP049090、PKF118-310、PKF115-584 和 ZTM000990)。此外,β-連環蛋白水平也可以通過靶向Wnt通路的上游組分以及β-連環蛋白破壞複合物來影響。[74]額外的N端結合口袋對於Wnt靶基因活化(BCL9募集所需)也很重要。例如,ARM結構域的這個位點可以被鼠尾草酸作為藥理學目標。[75]該「輔助」站點是藥物開發的另一個有吸引力的目標。[76]儘管進行了深入的臨床前研究,但尚無β-連環蛋白抑制劑可用作治療劑。然而,它的功能可以通過基於獨立驗證的siRNA敲低來進一步檢查。[77]另一種減少β-連環蛋白核積累的治療方法是通過抑制半乳糖凝集素3。[78]半乳糖凝集素3抑制劑GR-MD-02目前正在與FDA批准的伊匹木單抗劑量聯合用於晚期黑色素瘤患者的臨床試驗。[79]蛋白質BCL9和BCL9L已被提議作為呈現過度活化的Wnt信號傳導的結腸直腸癌的治療靶標,因為它們的缺失不會干擾正常的體內平衡,但會強烈影響遠端轉移行為。[80]
在胎兒酒精譜系障礙中的作用
[編輯]乙醇引起的β-連環蛋白不穩定是兩種已知途徑之一,酒精暴露會導致胎兒酒精譜系障礙(另一種是乙醇誘導的葉酸缺乏症)。乙醇通過G蛋白依賴性途徑導致β-連環蛋白失穩,其中活化的磷脂酶Cβ將4,5-二磷酸磷脂酰肌醇水解為二酸甘油酯和1,4,5-三磷酸肌醇。可溶性1,4,5-三磷酸肌醇觸發鈣從內質網釋放。這種細胞質鈣的突然增加會活化鈣離子/鈣調素依賴性蛋白激酶 (CaMK)。活化的CaMK通過一個表徵不佳的機制使β-連環蛋白不穩定,但這可能涉及CaMK對β-連環蛋白的磷酸化。 β-連環蛋白轉錄程式(正常神經嵴細胞發育所需)因此被抑制,導致神經嵴細胞過早凋亡(細胞死亡)。[81]
相互作用
[編輯]β-連環蛋白已被證明能與以下物質相互作用:
- APC[82][83][84][85][86][87][88][89]
- AXIN1[90][91]
- CBY1[92]
- CDH1[25][83][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113]
- CDH2[52][114][115]
- CDH3[112][116]
- CDH5[117][118]
- CDK5R1[119]
- CHUK[120]
- CTNND1[83][98]
- CTNNA1[94][103][121][122][123]
- EGFR[98][107][124]
- ESR1[66]
- FHL2[125]
- GSK3B[85][126]
- HER2/neu[99][124][127]
- HNF4A[128]
- IKK2[120]
- LEF1[129][130][131][132]包括轉基因的[133]
- MAGI1[108]
- MUC1[100][134][135][136][137][138][139]
- NR5A1[140][141]
- PCAF[142]
- PHF17[143]
- PTPN14[144]
- PTPRF[99][145]
- PTPRK[146]
- PTPRT[147]
- PTPRU[148][149][150]
- PSEN1[151][152][153]
- PTK7[154]
- RUVBL1[155]
- SMAD7[129]
- SMARCA4[156]
- SLC9A3R1[102]
- USP9X[157]
- XIRP1[158]
- 雄激素受體[159][160][161][162][128][163]
- 埃默里蛋白[164][165]
- 斑珠蛋白[83][98]
參見
[編輯]參考文獻
[編輯]- ^ 與CTNNB1相關的疾病;在維基數據上查看/編輯參考.
- ^ 2.0 2.1 2.2 GRCh38: Ensembl release 89: ENSG00000168036 - Ensembl, May 2017
- ^ 3.0 3.1 3.2 GRCm38: Ensembl release 89: ENSMUSG00000006932 - Ensembl, May 2017
- ^ Human PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mouse PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Kraus C, Liehr T, Hülsken J, Behrens J, Birchmeier W, Grzeschik KH, Ballhausen WG. Localization of the human beta-catenin gene (CTNNB1) to 3p21: a region implicated in tumor development. Genomics. September 1994, 23 (1): 272–274. PMID 7829088. doi:10.1006/geno.1994.1493.
- ^ MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental Cell. July 2009, 17 (1): 9–26. PMC 2861485
 . PMID 19619488. doi:10.1016/j.devcel.2009.06.016.
. PMID 19619488. doi:10.1016/j.devcel.2009.06.016.
- ^ Peifer M, Rauskolb C, Williams M, Riggleman B, Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development. April 1991, 111 (4): 1029–1043. PMID 1879348. doi:10.1242/dev.111.4.1029.
- ^ Noordermeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature. January 1994, 367 (6458): 80–83. Bibcode:1994Natur.367...80N. PMID 7906389. S2CID 4275610. doi:10.1038/367080a0.
- ^ Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. March 1994, 76 (5): 789–791. PMID 7907279. S2CID 26528190. doi:10.1016/0092-8674(94)90353-0.
- ^ Morin PJ. beta-catenin signaling and cancer. BioEssays. December 1999, 21 (12): 1021–1030. PMID 10580987. S2CID 86240312. doi:10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P.
- ^ McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. November 1991, 254 (5036): 1359–1361. Bibcode:1991Sci...254.1359M. PMID 1962194. doi:10.1126/science.1962194.
- ^ Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends in Genetics. September 1993, 9 (9): 317–321. PMID 8236461. doi:10.1016/0168-9525(93)90250-l.
- ^ Gottardi CJ, Peifer M. Terminal regions of beta-catenin come into view. Structure. March 2008, 16 (3): 336–338. PMC 2329800
 . PMID 18334207. doi:10.1016/j.str.2008.02.005.
. PMID 18334207. doi:10.1016/j.str.2008.02.005.
- ^ Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, Xu W. Crystal structure of a full-length beta-catenin. Structure. March 2008, 16 (3): 478–487. PMC 4267759
 . PMID 18334222. doi:10.1016/j.str.2007.12.021.
. PMID 18334222. doi:10.1016/j.str.2007.12.021.
- ^ Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. The Journal of Biological Chemistry. April 2007, 282 (15): 11221–11229. PMC 1850976
 . PMID 17287208. doi:10.1074/jbc.M611871200
. PMID 17287208. doi:10.1074/jbc.M611871200  .
.
- ^ Söderholm S, Cantù C. The WNT/β-catenin dependent transcription: A tissue-specific business. WIREs Mechanisms of Disease. May 2021, 13 (3): e1511. PMC 9285942
 . PMID 33085215. doi:10.1002/wsbm.1511
. PMID 33085215. doi:10.1002/wsbm.1511  .
.
- ^ Vleminckx K, Kemler R, Hecht A. The C-terminal transactivation domain of beta-catenin is necessary and sufficient for signaling by the LEF-1/beta-catenin complex in Xenopus laevis. Mechanisms of Development. March 1999, 81 (1–2): 65–74. PMID 10330485. S2CID 15086656. doi:10.1016/s0925-4773(98)00225-1
 .
.
- ^ Sadot E, Simcha I, Iwai K, Ciechanover A, Geiger B, Ben-Ze'ev A. Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system. Oncogene. April 2000, 19 (16): 1992–2001. PMID 10803460. doi:10.1038/sj.onc.1203519
 .
.
- ^ Aktary Z, Pasdar M. Plakoglobin: role in tumorigenesis and metastasis. International Journal of Cell Biology. 2012, 2012: 189521. PMC 3312339
 . PMID 22481945. doi:10.1155/2012/189521
. PMID 22481945. doi:10.1155/2012/189521  .
.
- ^ Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. Journal of Cell Science. October 2007, 120 (Pt 19): 3337–3344. PMID 17881495. doi:10.1242/jcs.013771
 .
.
- ^ Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta-catenin/Tcf complex. Cell. December 2000, 103 (6): 885–896. PMID 11136974. S2CID 16865193. doi:10.1016/S0092-8674(00)00192-6
 .
.
- ^ Graham TA, Ferkey DM, Mao F, Kimelman D, Xu W. Tcf4 can specifically recognize beta-catenin using alternative conformations. Nature Structural Biology. December 2001, 8 (12): 1048–1052. PMID 11713475. S2CID 33878077. doi:10.1038/nsb718.
- ^ Poy F, Lepourcelet M, Shivdasani RA, Eck MJ. Structure of a human Tcf4-beta-catenin complex. Nature Structural Biology. December 2001, 8 (12): 1053–1057. PMID 11713476. S2CID 24798619. doi:10.1038/nsb720.
- ^ 25.0 25.1 Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. May 2001, 105 (3): 391–402. PMID 11348595. S2CID 364223. doi:10.1016/S0092-8674(01)00330-0
 .
.
- ^ 26.0 26.1 Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes & Development. November 2003, 17 (22): 2753–2764. PMC 280624
 . PMID 14600025. doi:10.1101/gad.1142603.
. PMID 14600025. doi:10.1101/gad.1142603.
- ^ 27.0 27.1 Minde DP, Anvarian Z, Rüdiger SG, Maurice MM. Messing up disorder: how do missense mutations in the tumor suppressor protein APC lead to cancer?. Molecular Cancer. August 2011, 10 (1): 101. PMC 3170638
 . PMID 21859464. doi:10.1186/1476-4598-10-101.
. PMID 21859464. doi:10.1186/1476-4598-10-101.
- ^ Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. April 2002, 109 (1): 47–60. PMID 11955446. S2CID 16720801. doi:10.1016/S0092-8674(02)00679-7.
- ^ Pokutta S, Weis WI. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Molecular Cell. March 2000, 5 (3): 533–543. PMID 10882138. doi:10.1016/S1097-2765(00)80447-5
 .
.
- ^ Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a beta-catenin/BCL9/Tcf4 complex. Molecular Cell. October 2006, 24 (2): 293–300. PMID 17052462. doi:10.1016/j.molcel.2006.09.001
 .
.
- ^ Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes & Development. September 2004, 18 (18): 2225–2230. PMC 517514
 . PMID 15371335. doi:10.1101/gad.317604.
. PMID 15371335. doi:10.1101/gad.317604.
- ^ Liu J, Xing Y, Hinds TR, Zheng J, Xu W. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. Journal of Molecular Biology. June 2006, 360 (1): 133–144. PMID 16753179. doi:10.1016/j.jmb.2006.04.064.
- ^ Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. December 2006, 25 (57): 7482–7491. PMID 17143292. doi:10.1038/sj.onc.1210055
 .
.
- ^ Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proceedings of the National Academy of Sciences of the United States of America. February 2011, 108 (5): 1937–1942. Bibcode:2011PNAS..108.1937F. PMC 3033301
 . PMID 21245303. doi:10.1073/pnas.1017063108
. PMID 21245303. doi:10.1073/pnas.1017063108  .
.
- ^ Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling--two contrasting models. Journal of Cell Science. November 2011, 124 (Pt 21): 3537–3544. PMID 22083140. doi:10.1242/jcs.091991
 .
.
- ^ 36.0 36.1 Brembeck FH, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Current Opinion in Genetics & Development. February 2006, 16 (1): 51–59. PMID 16377174. doi:10.1016/j.gde.2005.12.007.
- ^ Entrez Gene: catenin (cadherin-associated protein). [2022-10-27]. (原始內容存檔於2010-03-07).
- ^ Bush M, Alhanshali BM, Qian S, Stanley CB, Heller WT, Matsui T, et al. An ensemble of flexible conformations underlies mechanotransduction by the cadherin-catenin adhesion complex. Proceedings of the National Academy of Sciences of the United States of America. October 2019, 116 (43): 21545–21555. PMC 6815173
 . PMID 31591245. doi:10.1073/pnas.1911489116
. PMID 31591245. doi:10.1073/pnas.1911489116  .
.
- ^ Röper JC, Mitrossilis D, Stirnemann G, Waharte F, Brito I, Fernandez-Sanchez ME, et al. The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. eLife. July 2018, 7. PMC 6053302
 . PMID 30024850. doi:10.7554/eLife.33381.
. PMID 30024850. doi:10.7554/eLife.33381.
- ^ Farago B, Nicholl ID, Wang S, Cheng X, Callaway DJ, Bu Z. Activated nanoscale actin-binding domain motion in the catenin-cadherin complex revealed by neutron spin echo spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. March 2021, 118 (13): e2025012118. Bibcode:2021PNAS..11825012F. PMC 8020631
 . PMID 33753508. doi:10.1073/pnas.2025012118.
. PMID 33753508. doi:10.1073/pnas.2025012118.
- ^ Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochemical Society Transactions. April 2008, 36 (Pt 2): 149–155. PMC 3368607
 . PMID 18363555. doi:10.1042/BST0360149.
. PMID 18363555. doi:10.1042/BST0360149.
- ^ Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Current Biology. January 2005, 15 (2): R64–R67. PMID 15668160. S2CID 12352182. doi:10.1016/j.cub.2004.12.058
 .
.
- ^ 43.0 43.1 Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. November 1995, 121 (11): 3529–3537. PMID 8582267. doi:10.1242/dev.121.11.3529.
- ^ 44.0 44.1 Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. October 2011, 138 (20): 4341–4350. PMC 3177306
 . PMID 21903672. doi:10.1242/dev.066209.
. PMID 21903672. doi:10.1242/dev.066209.
- ^ Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mechanisms of Development. July 1996, 57 (2): 191–198. PMID 8843396. S2CID 12694740. doi:10.1016/0925-4773(96)00546-1
 .
.
- ^ Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. The Journal of Cell Biology. March 1997, 136 (5): 1123–1136. PMC 2132470
 . PMID 9060476. doi:10.1083/jcb.136.5.1123.
. PMID 9060476. doi:10.1083/jcb.136.5.1123.
- ^ Kelly GM, Erezyilmaz DF, Moon RT. Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mechanisms of Development. October 1995, 53 (2): 261–273. PMID 8562427. S2CID 14885037. doi:10.1016/0925-4773(95)00442-4
 .
.
- ^ Sawa H. Control of cell polarity and asymmetric division in C. elegans. Current Topics in Developmental Biology. 2012, 101: 55–76. ISBN 9780123945921. PMID 23140625. doi:10.1016/B978-0-12-394592-1.00003-X.
- ^ Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, et al. E-cadherin/β-catenin complex and the epithelial barrier. Journal of Biomedicine & Biotechnology. 2011, 2011: 567305. PMC 3191826
 . PMID 22007144. doi:10.1155/2011/567305
. PMID 22007144. doi:10.1155/2011/567305  .
.
- ^ Zelarayan L, Gehrke C, Bergmann MW. Role of beta-catenin in adult cardiac remodeling. Cell Cycle. September 2007, 6 (17): 2120–2126. PMID 17786052. doi:10.4161/cc.6.17.4632
 .
.
- ^ Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Developmental Cell. August 2002, 3 (2): 171–181. PMID 12194849. doi:10.1016/s1534-5807(02)00206-x
 .
.
- ^ 52.0 52.1 Hertig CM, Butz S, Koch S, Eppenberger-Eberhardt M, Kemler R, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. Journal of Cell Science. January 1996,. 109 ( Pt 1) (1): 11–20. PMID 8834786. doi:10.1242/jcs.109.1.11.
- ^ Wheeler MA, Warley A, Roberts RG, Ehler E, Ellis JA. Identification of an emerin-beta-catenin complex in the heart important for intercalated disc architecture and beta-catenin localisation. Cellular and Molecular Life Sciences. March 2010, 67 (5): 781–796. PMID 19997769. S2CID 27205170. doi:10.1007/s00018-009-0219-8.
- ^ Wang X, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. Journal of Molecular and Cellular Cardiology. February 1999, 31 (2): 333–343. PMID 10093046. doi:10.1006/jmcc.1998.0886.
- ^ Yoshida M, Ohkusa T, Nakashima T, Takanari H, Yano M, Takemura G, et al. Alterations in adhesion junction precede gap junction remodelling during the development of heart failure in cardiomyopathic hamsters. Cardiovascular Research. October 2011, 92 (1): 95–105. PMID 21693625. doi:10.1093/cvr/cvr182
 .
.
- ^ Hahn JY, Cho HJ, Bae JW, Yuk HS, Kim KI, Park KW, et al. Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. The Journal of Biological Chemistry. October 2006, 281 (41): 30979–30989. PMID 16920707. doi:10.1074/jbc.M603916200
 .
.
- ^ Zheng Q, Chen P, Xu Z, Li F, Yi XP. Expression and redistribution of β-catenin in the cardiac myocytes of left ventricle of spontaneously hypertensive rat. Journal of Molecular Histology. October 2013, 44 (5): 565–573. PMID 23591738. S2CID 18997718. doi:10.1007/s10735-013-9507-6.
- ^ Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, et al. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circulation Research. May 2007, 100 (9): 1353–1362. PMID 17413044. doi:10.1161/01.RES.0000266605.63681.5a
 .
.
- ^ Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, et al. The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Molecular and Cellular Biology. June 2006, 26 (12): 4462–4473. PMC 1489123
 . PMID 16738313. doi:10.1128/MCB.02157-05.
. PMID 16738313. doi:10.1128/MCB.02157-05.
- ^ Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, et al. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proceedings of the National Academy of Sciences of the United States of America. April 2003, 100 (8): 4610–4615. Bibcode:2003PNAS..100.4610H. PMC 153603
 . PMID 12668767. doi:10.1073/pnas.0835895100
. PMID 12668767. doi:10.1073/pnas.0835895100  .
.
- ^ Hirschy A, Croquelois A, Perriard E, Schoenauer R, Agarkova I, Hoerstrup SP, et al. Stabilised beta-catenin in postnatal ventricular myocardium leads to dilated cardiomyopathy and premature death (PDF). Basic Research in Cardiology. September 2010, 105 (5): 597–608 [2022-10-29]. PMID 20376467. S2CID 21789076. doi:10.1007/s00395-010-0101-8. (原始內容存檔 (PDF)於2022-10-17).
- ^ Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL. Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {betacatenin signaling}-. Molecular and Cellular Biology. March 2011, 31 (6): 1134–1144. PMC 3067899
 . PMID 21245375. doi:10.1128/MCB.01025-10.
. PMID 21245375. doi:10.1128/MCB.01025-10.
- ^ Swope D, Cheng L, Gao E, Li J, Radice GL. Loss of cadherin-binding proteins β-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis. Molecular and Cellular Biology. March 2012, 32 (6): 1056–1067. PMC 3295003
 . PMID 22252313. doi:10.1128/MCB.06188-11.
. PMID 22252313. doi:10.1128/MCB.06188-11.
- ^ 64.0 64.1 Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. December 2014, 516 (7529): 51–55. Bibcode:2014Natur.516...51D. PMC 4257892
 . PMID 25383518. doi:10.1038/nature13976.
. PMID 25383518. doi:10.1038/nature13976.
- ^ Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc?. Trends in Cardiovascular Medicine. January 2003, 13 (1): 30–38. PMID 12554098. doi:10.1016/s1050-1738(02)00209-8.
- ^ 66.0 66.1 Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, et al. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB Journal. May 2006, 20 (7): 926–934. PMID 16675850. S2CID 2246390. doi:10.1096/fj.05-5148com.
- ^ Cantù C, Felker A, Zimmerli D, Prummel KD, Cabello EM, Chiavacci E, et al. Mutations in Bcl9 and Pygo genes cause congenital heart defects by tissue-specific perturbation of Wnt/β-catenin signaling. Genes & Development. November 2018, 32 (21–22): 1443–1458. PMC 6217730
 . PMID 30366904. doi:10.1101/gad.315531.118
. PMID 30366904. doi:10.1101/gad.315531.118  .
.
- ^ Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Research. January 2011, 39 (Database issue): D945–D950. PMC 3013785
 . PMID 20952405. doi:10.1093/nar/gkq929.
. PMID 20952405. doi:10.1093/nar/gkq929.
- ^ Saldanha G, Ghura V, Potter L, Fletcher A. Nuclear beta-catenin in basal cell carcinoma correlates with increased proliferation. The British Journal of Dermatology. July 2004, 151 (1): 157–164. PMID 15270885. S2CID 31114274. doi:10.1111/j.1365-2133.2004.06048.x.
- ^ Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nature Reviews. Urology. August 2012, 9 (8): 418–428. PMID 22710668. S2CID 22945223. doi:10.1038/nrurol.2012.116.
- ^ Hassanein AM, Glanz SM, Kessler HP, Eskin TA, Liu C. beta-Catenin is expressed aberrantly in tumors expressing shadow cells. Pilomatricoma, craniopharyngioma, and calcifying odontogenic cyst. American Journal of Clinical Pathology. November 2003, 120 (5): 732–736. PMID 14608900. doi:10.1309/EALEG7LD6W7167PX
 .
.
- ^ Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. Journal of Clinical Oncology. November 2005, 23 (31): 7951–7957. PMID 16258095. doi:10.1200/JCO.2005.01.5479.
- ^ Kobayashi M, Honma T, Matsuda Y, Suzuki Y, Narisawa R, Ajioka Y, Asakura H. Nuclear translocation of beta-catenin in colorectal cancer. British Journal of Cancer. May 2000, 82 (10): 1689–1693. PMC 2374509
 . PMID 10817505. doi:10.1054/bjoc.1999.1112.
. PMID 10817505. doi:10.1054/bjoc.1999.1112.
- ^ Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Current Pharmaceutical Design. 2013, 19 (4): 634–664. PMC 3529405
 . PMID 23016862. doi:10.2174/1381612811306040634.
. PMID 23016862. doi:10.2174/1381612811306040634.
- ^ de la Roche M, Rutherford TJ, Gupta D, Veprintsev DB, Saxty B, Freund SM, Bienz M. An intrinsically labile α-helix abutting the BCL9-binding site of β-catenin is required for its inhibition by carnosic acid. Nature Communications. February 2012, 3 (2): 680. Bibcode:2012NatCo...3..680D. PMC 3293410
 . PMID 22353711. doi:10.1038/ncomms1680.
. PMID 22353711. doi:10.1038/ncomms1680.
- ^ Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao JJ, Mani M, et al. Targeted disruption of the BCL9/β-catenin complex inhibits oncogenic Wnt signaling. Science Translational Medicine. August 2012, 4 (148): 148ra117. PMC 3631420
 . PMID 22914623. doi:10.1126/scitranslmed.3003808.
. PMID 22914623. doi:10.1126/scitranslmed.3003808.
- ^ Munkácsy G, Sztupinszki Z, Herman P, Bán B, Pénzváltó Z, Szarvas N, Győrffy B. Validation of RNAi Silencing Efficiency Using Gene Array Data shows 18.5% Failure Rate across 429 Independent Experiments. Molecular Therapy. Nucleic Acids. September 2016, 5 (9): e366. PMC 5056990
 . PMID 27673562. doi:10.1038/mtna.2016.66.
. PMID 27673562. doi:10.1038/mtna.2016.66.
- ^ Cao Z, Hao Z, Xin M, Yu L, Wang L, Zhang Y, et al. Endogenous and exogenous galectin-3 promote the adhesion of tumor cells with low expression of MUC1 to HUVECs through upregulation of N-cadherin and CD44. Laboratory Investigation; A Journal of Technical Methods and Pathology. December 2018, 98 (12): 1642–1656. PMID 30171204. S2CID 52139917. doi:10.1038/s41374-018-0119-3
 .
.
- ^ Clinical trial number NCT02117362 for "Galectin Inhibitor (GR-MD-02) and Ipilimumab in Patients With Metastatic Melanoma" at ClinicalTrials.gov
- ^ Moor AE, Anderle P, Cantù C, Rodriguez P, Wiedemann N, Baruthio F, et al. BCL9/9L-β-catenin Signaling is Associated With Poor Outcome in Colorectal Cancer. EBioMedicine. December 2015, 2 (12): 1932–1943. PMC 4703711
 . PMID 26844272. doi:10.1016/j.ebiom.2015.10.030.
. PMID 26844272. doi:10.1016/j.ebiom.2015.10.030.
- ^ Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. Calcium-mediated repression of β-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Research. Part A, Clinical and Molecular Teratology. July 2011, 91 (7): 591–602. PMC 4827605
 . PMID 21630427. doi:10.1002/bdra.20833.
. PMID 21630427. doi:10.1002/bdra.20833.
- ^ Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. December 1993, 262 (5140): 1734–1737. Bibcode:1993Sci...262.1734S. PMID 8259519. doi:10.1126/science.8259519.
- ^ 83.0 83.1 83.2 83.3 Kucerová D, Sloncová E, Tuhácková Z, Vojtechová M, Sovová V. Expression and interaction of different catenins in colorectal carcinoma cells. International Journal of Molecular Medicine. December 2001, 8 (6): 695–698. PMID 11712088. doi:10.3892/ijmm.8.6.695.
- ^ Tickenbrock L, Kössmeier K, Rehmann H, Herrmann C, Müller O. Differences between the interaction of beta-catenin with non-phosphorylated and single-mimicked phosphorylated 20-amino acid residue repeats of the APC protein. Journal of Molecular Biology. March 2003, 327 (2): 359–367. PMID 12628243. doi:10.1016/S0022-2836(03)00144-X.
- ^ 85.0 85.1 Davies G, Jiang WG, Mason MD. The interaction between beta-catenin, GSK3beta and APC after motogen induced cell-cell dissociation, and their involvement in signal transduction pathways in prostate cancer. International Journal of Oncology. April 2001, 18 (4): 843–847. PMID 11251183. doi:10.3892/ijo.18.4.843.
- ^ Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nature Cell Biology. September 2001, 3 (9): 793–801. PMID 11533658. S2CID 664553. doi:10.1038/ncb0901-793.
- ^ Homma MK, Li D, Krebs EG, Yuasa Y, Homma Y. Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein. Proceedings of the National Academy of Sciences of the United States of America. April 2002, 99 (9): 5959–5964. Bibcode:2002PNAS...99.5959K. PMC 122884
 . PMID 11972058. doi:10.1073/pnas.092143199
. PMID 11972058. doi:10.1073/pnas.092143199  .
.
- ^ Satoh K, Yanai H, Senda T, Kohu K, Nakamura T, Okumura N, et al. DAP-1, a novel protein that interacts with the guanylate kinase-like domains of hDLG and PSD-95. Genes to Cells. June 1997, 2 (6): 415–424. PMID 9286858. S2CID 8934092. doi:10.1046/j.1365-2443.1997.1310329.x
 .
.
- ^ Eklof Spink K, Fridman SG, Weis WI. Molecular mechanisms of beta-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-beta-catenin complex. The EMBO Journal. November 2001, 20 (22): 6203–6212. PMC 125720
 . PMID 11707392. doi:10.1093/emboj/20.22.6203.
. PMID 11707392. doi:10.1093/emboj/20.22.6203.
- ^ Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes to Cells. June 1998, 3 (6): 395–403. PMID 9734785. S2CID 10875463. doi:10.1046/j.1365-2443.1998.00198.x
 .
.
- ^ Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Regulation of the Wnt signaling pathway by disabled-2 (Dab2). The EMBO Journal. June 2003, 22 (12): 3084–3094. PMC 162138
 . PMID 12805222. doi:10.1093/emboj/cdg286.
. PMID 12805222. doi:10.1093/emboj/cdg286.
- ^ Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. April 2003, 422 (6934): 905–909. Bibcode:2003Natur.422..905T. PMID 12712206. S2CID 4418716. doi:10.1038/nature01570.
- ^ Davies G, Jiang WG, Mason MD. HGF/SF modifies the interaction between its receptor c-Met, and the E-cadherin/catenin complex in prostate cancer cells. International Journal of Molecular Medicine. April 2001, 7 (4): 385–388. PMID 11254878. doi:10.3892/ijmm.7.4.385.
- ^ 94.0 94.1 Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, et al. A truncated beta-catenin disrupts the interaction between E-cadherin and alpha-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Research. December 1994, 54 (23): 6282–6287. PMID 7954478.
- ^ Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. The Journal of Biological Chemistry. December 1997, 272 (51): 32448–32453. PMID 9405455. doi:10.1074/jbc.272.51.32448
 .
.
- ^ Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. The Journal of Cell Biology. July 1995, 130 (2): 461–471. PMC 2199929
 . PMID 7542250. doi:10.1083/jcb.130.2.461.
. PMID 7542250. doi:10.1083/jcb.130.2.461.
- ^ Jiang MC, Liao CF, Tai CC. CAS/CSE 1 stimulates E-cadhrin-dependent cell polarity in HT-29 human colon epithelial cells. Biochemical and Biophysical Research Communications. June 2002, 294 (4): 900–905. PMID 12061792. doi:10.1016/S0006-291X(02)00551-X.
- ^ 98.0 98.1 98.2 98.3 Hazan RB, Norton L. The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. The Journal of Biological Chemistry. April 1998, 273 (15): 9078–9084. PMID 9535896. doi:10.1074/jbc.273.15.9078
 .
.
- ^ 99.0 99.1 99.2 Bonvini P, An WG, Rosolen A, Nguyen P, Trepel J, Garcia de Herreros A, et al. Geldanamycin abrogates ErbB2 association with proteasome-resistant beta-catenin in melanoma cells, increases beta-catenin-E-cadherin association, and decreases beta-catenin-sensitive transcription. Cancer Research. February 2001, 61 (4): 1671–1677. PMID 11245482.
- ^ 100.0 100.1 Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Molecular and Cellular Biology. December 1998, 18 (12): 7216–7224. PMC 109303
 . PMID 9819408. doi:10.1128/mcb.18.12.7216.
. PMID 9819408. doi:10.1128/mcb.18.12.7216.
- ^ Wendeler MW, Praus M, Jung R, Hecking M, Metzig C, Gessner R. Ksp-cadherin is a functional cell-cell adhesion molecule related to LI-cadherin. Experimental Cell Research. April 2004, 294 (2): 345–355. PMID 15023525. doi:10.1016/j.yexcr.2003.11.022.
- ^ 102.0 102.1 Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S. EBP50, a beta-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology. July 2003, 38 (1): 178–186. PMID 12830000. S2CID 10325091. doi:10.1053/jhep.2003.50270
 .
.
- ^ 103.0 103.1 Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, Duñach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Molecular and Cellular Biology. April 2003, 23 (7): 2287–2297. PMC 150740
 . PMID 12640114. doi:10.1128/MCB.23.7.2287-2297.2003.
. PMID 12640114. doi:10.1128/MCB.23.7.2287-2297.2003.
- ^ Kang JS, Feinleib JL, Knox S, Ketteringham MA, Krauss RS. Promyogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proceedings of the National Academy of Sciences of the United States of America. April 2003, 100 (7): 3989–3994. Bibcode:2003PNAS..100.3989K. PMC 153035
 . PMID 12634428. doi:10.1073/pnas.0736565100
. PMID 12634428. doi:10.1073/pnas.0736565100  .
.
- ^ Oneyama C, Nakano H, Sharma SV. UCS15A, a novel small molecule, SH3 domain-mediated protein-protein interaction blocking drug. Oncogene. March 2002, 21 (13): 2037–2050. PMID 11960376. doi:10.1038/sj.onc.1205271
 .
.
- ^ Navarro P, Lozano E, Cano A. Expression of E- or P-cadherin is not sufficient to modify the morphology and the tumorigenic behavior of murine spindle carcinoma cells. Possible involvement of plakoglobin. Journal of Cell Science. August 1993,. 105 ( Pt 4) (4): 923–934. PMID 8227214. doi:10.1242/jcs.105.4.923. hdl:10261/78716
 .
.
- ^ 107.0 107.1 Takahashi K, Suzuki K, Tsukatani Y. Induction of tyrosine phosphorylation and association of beta-catenin with EGF receptor upon tryptic digestion of quiescent cells at confluence. Oncogene. July 1997, 15 (1): 71–78. PMID 9233779. doi:10.1038/sj.onc.1201160
 .
.
- ^ 108.0 108.1 Dobrosotskaya IY, James GL. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochemical and Biophysical Research Communications. April 2000, 270 (3): 903–909. PMID 10772923. doi:10.1006/bbrc.2000.2471.
- ^ Geng L, Burrow CR, Li HP, Wilson PD. Modification of the composition of polycystin-1 multiprotein complexes by calcium and tyrosine phosphorylation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. December 2000, 1535 (1): 21–35. PMID 11113628. doi:10.1016/S0925-4439(00)00079-X
 .
.
- ^ Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, et al. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. The Journal of Cell Biology. March 1995, 128 (5): 949–957. PMC 2120395
 . PMID 7876318. doi:10.1083/jcb.128.5.949.
. PMID 7876318. doi:10.1083/jcb.128.5.949.
- ^ Rao RK, Basuroy S, Rao VU, Karnaky KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. The Biochemical Journal. December 2002, 368 (Pt 2): 471–481. PMC 1222996
 . PMID 12169098. doi:10.1042/BJ20011804.
. PMID 12169098. doi:10.1042/BJ20011804.
- ^ 112.0 112.1 Schmeiser K, Grand RJ. The fate of E- and P-cadherin during the early stages of apoptosis. Cell Death and Differentiation. April 1999, 6 (4): 377–386. PMID 10381631. doi:10.1038/sj.cdd.4400504
 .
.
- ^ Pai R, Dunlap D, Qing J, Mohtashemi I, Hotzel K, French DM. Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating beta-catenin signaling. Cancer Research. July 2008, 68 (13): 5086–5095. PMID 18593907. doi:10.1158/0008-5472.CAN-07-2325
 .
.
- ^ Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, et al. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. Journal of Cell Science. December 2003, 116 (Pt 24): 4985–4995. PMID 14625392. doi:10.1242/jcs.00815
 .
.
- ^ Wahl JK, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. The Journal of Biological Chemistry. May 2003, 278 (19): 17269–17276. PMID 12604612. doi:10.1074/jbc.M211452200
 .
.
- ^ Klingelhöfer J, Troyanovsky RB, Laur OY, Troyanovsky S. Amino-terminal domain of classic cadherins determines the specificity of the adhesive interactions. Journal of Cell Science. August 2000,. 113 ( Pt 16) (16): 2829–2836. PMID 10910767. doi:10.1242/jcs.113.16.2829.
- ^ Lewalle JM, Bajou K, Desreux J, Mareel M, Dejana E, Noël A, Foidart JM. Alteration of interendothelial adherens junctions following tumor cell-endothelial cell interaction in vitro. Experimental Cell Research. December 1997, 237 (2): 347–356. PMID 9434630. doi:10.1006/excr.1997.3799. hdl:2268/61990.
- ^ Shasby DM, Ries DR, Shasby SS, Winter MC. Histamine stimulates phosphorylation of adherens junction proteins and alters their link to vimentin. American Journal of Physiology. Lung Cellular and Molecular Physiology. June 2002, 282 (6): L1330–L1338. CiteSeerX 10.1.1.1000.5266
 . PMID 12003790. doi:10.1152/ajplung.00329.2001.
. PMID 12003790. doi:10.1152/ajplung.00329.2001.
- ^ Kesavapany S, Lau KF, McLoughlin DM, Brownlees J, Ackerley S, Leigh PN, et al. p35/cdk5 binds and phosphorylates beta-catenin and regulates beta-catenin/presenilin-1 interaction. The European Journal of Neuroscience. January 2001, 13 (2): 241–247. PMID 11168528. doi:10.1046/j.1460-9568.2001.01376.x.
- ^ 120.0 120.1 Lamberti C, Lin KM, Yamamoto Y, Verma U, Verma IM, Byers S, Gaynor RB. Regulation of beta-catenin function by the IkappaB kinases. The Journal of Biological Chemistry. November 2001, 276 (45): 42276–42286. PMID 11527961. doi:10.1074/jbc.M104227200
 .
.
- ^ Roe S, Koslov ER, Rimm DL. A mutation in alpha-catenin disrupts adhesion in clone A cells without perturbing its actin and beta-catenin binding activity. Cell Adhesion and Communication. June 1998, 5 (4): 283–296. PMID 9762469. doi:10.3109/15419069809040298
 .
.
- ^ Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. Journal of Cell Science. December 1994,. 107 ( Pt 12) (12): 3655–3663. PMID 7706414. doi:10.1242/jcs.107.12.3655.
- ^ Reuver SM, Garner CC. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. Journal of Cell Science. April 1998,. 111 ( Pt 8) (8): 1071–1080. PMID 9512503. doi:10.1242/jcs.111.8.1071.
- ^ 124.0 124.1 Schroeder JA, Adriance MC, McConnell EJ, Thompson MC, Pockaj B, Gendler SJ. ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. The Journal of Biological Chemistry. June 2002, 277 (25): 22692–22698. PMID 11950845. doi:10.1074/jbc.M201975200
 .
.
- ^ Wei Y, Renard CA, Labalette C, Wu Y, Lévy L, Neuveut C, et al. Identification of the LIM protein FHL2 as a coactivator of beta-catenin. The Journal of Biological Chemistry. February 2003, 278 (7): 5188–5194. PMID 12466281. doi:10.1074/jbc.M207216200
 .
.
- ^ Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Molecular and Cellular Biology. June 1999, 19 (6): 4414–4422. PMC 104400
 . PMID 10330181. doi:10.1128/mcb.19.6.4414.
. PMID 10330181. doi:10.1128/mcb.19.6.4414.
- ^ Kanai Y, Ochiai A, Shibata T, Oyama T, Ushijima S, Akimoto S, Hirohashi S. c-erbB-2 gene product directly associates with beta-catenin and plakoglobin. Biochemical and Biophysical Research Communications. March 1995, 208 (3): 1067–1072. PMID 7702605. doi:10.1006/bbrc.1995.1443.
- ^ 128.0 128.1 Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: a means for inhibition of the Tcf signaling axis. Oncogene. August 2003, 22 (36): 5602–5613. PMID 12944908. doi:10.1038/sj.onc.1206802
 .
.
- ^ 129.0 129.1 Edlund S, Lee SY, Grimsby S, Zhang S, Aspenström P, Heldin CH, Landström M. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Molecular and Cellular Biology. February 2005, 25 (4): 1475–1488. PMC 548008
 . PMID 15684397. doi:10.1128/MCB.25.4.1475-1488.2005.
. PMID 15684397. doi:10.1128/MCB.25.4.1475-1488.2005.
- ^ Grueneberg DA, Pablo L, Hu KQ, August P, Weng Z, Papkoff J. A functional screen in human cells identifies UBF2 as an RNA polymerase II transcription factor that enhances the beta-catenin signaling pathway. Molecular and Cellular Biology. June 2003, 23 (11): 3936–3950. PMC 155208
 . PMID 12748295. doi:10.1128/MCB.23.11.3936-3950.2003.
. PMID 12748295. doi:10.1128/MCB.23.11.3936-3950.2003.
- ^ Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. August 1996, 382 (6592): 638–642. Bibcode:1996Natur.382..638B. PMID 8757136. S2CID 4369341. doi:10.1038/382638a0.
- ^ Labbé E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proceedings of the National Academy of Sciences of the United States of America. July 2000, 97 (15): 8358–8363. Bibcode:2000PNAS...97.8358L. PMC 26952
 . PMID 10890911. doi:10.1073/pnas.150152697
. PMID 10890911. doi:10.1073/pnas.150152697  .
.
- ^ Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes & Development (Cold Spring Harbor Laboratory Press & The Genetics Society). May 2002, 16 (10): 1167–1181. PMID 12023297. S2CID 14376483. doi:10.1101/gad.976502.
In ... zebrafish, reporter transgenes containing the TOPFLASH promoter are expressed in certain Wnt-responsive cell types (...Dorsky et al. 2002).
- ^ Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. The Journal of Biological Chemistry. May 1997, 272 (19): 12492–12494. PMID 9139698. doi:10.1074/jbc.272.19.12492
 .
.
- ^ Durum SK, Aiello FB. Interleukin-7 induces MUC1. Cancer Biology & Therapy. 2003, 2 (2): 194–195. PMID 12750562. doi:10.4161/cbt.2.2.351
 .
.
- ^ Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. March 2003, 22 (9): 1324–1332. PMID 12618757. doi:10.1038/sj.onc.1206291
 .
.
- ^ Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. The Journal of Biological Chemistry. March 2001, 276 (9): 6061–6064. PMID 11152665. doi:10.1074/jbc.C000754200
 .
.
- ^ Ren J, Li Y, Kufe D. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. The Journal of Biological Chemistry. May 2002, 277 (20): 17616–17622. PMID 11877440. doi:10.1074/jbc.M200436200
 .
.
- ^ Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L, et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. The Journal of Biological Chemistry. September 2001, 276 (38): 35239–35242. PMID 11483589. doi:10.1074/jbc.C100359200
 .
.
- ^ Kennell JA, O'Leary EE, Gummow BM, Hammer GD, MacDougald OA. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with beta-catenin to coactivate C/EBPalpha and steroidogenic factor 1 transcription factors. Molecular and Cellular Biology. August 2003, 23 (15): 5366–5375. PMC 165725
 . PMID 12861022. doi:10.1128/MCB.23.15.5366-5375.2003.
. PMID 12861022. doi:10.1128/MCB.23.15.5366-5375.2003.
- ^ Mizusaki H, Kawabe K, Mukai T, Ariyoshi E, Kasahara M, Yoshioka H, et al. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Molecular Endocrinology. April 2003, 17 (4): 507–519. PMID 12554773. doi:10.1210/me.2002-0362
 .
.
- ^ Ge X, Jin Q, Zhang F, Yan T, Zhai Q. PCAF acetylates {betacatenin and improves its stability}-. Molecular Biology of the Cell. January 2009, 20 (1): 419–427. PMC 2613091
 . PMID 18987336. doi:10.1091/mbc.E08-08-0792.
. PMID 18987336. doi:10.1091/mbc.E08-08-0792.
- ^ Behrens J. One hit, two outcomes for VHL-mediated tumorigenesis. Nature Cell Biology. October 2008, 10 (10): 1127–1128. PMID 18830218. S2CID 36184371. doi:10.1038/ncb1008-1127.
- ^ Wadham C, Gamble JR, Vadas MA, Khew-Goodall Y. The protein tyrosine phosphatase Pez is a major phosphatase of adherens junctions and dephosphorylates beta-catenin. Molecular Biology of the Cell. June 2003, 14 (6): 2520–2529. PMC 194899
 . PMID 12808048. doi:10.1091/mbc.E02-09-0577.
. PMID 12808048. doi:10.1091/mbc.E02-09-0577.
- ^ Aicher B, Lerch MM, Müller T, Schilling J, Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. The Journal of Cell Biology. August 1997, 138 (3): 681–696. PMC 2141638
 . PMID 9245795. doi:10.1083/jcb.138.3.681.
. PMID 9245795. doi:10.1083/jcb.138.3.681.
- ^ Fuchs M, Müller T, Lerch MM, Ullrich A. Association of human protein-tyrosine phosphatase kappa with members of the armadillo family. The Journal of Biological Chemistry. July 1996, 271 (28): 16712–16719. PMID 8663237. doi:10.1074/jbc.271.28.16712
 .
.
- ^ Besco JA, Hooft van Huijsduijnen R, Frostholm A, Rotter A. Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT). Brain Research. October 2006, 1116 (1): 50–57. PMID 16973135. S2CID 23343123. doi:10.1016/j.brainres.2006.07.122.
- ^ Wang B, Kishihara K, Zhang D, Hara H, Nomoto K. Molecular cloning and characterization of a novel human receptor protein tyrosine phosphatase gene, hPTP-J: down-regulation of gene expression by PMA and calcium ionophore in Jurkat T lymphoma cells. Biochemical and Biophysical Research Communications. February 1997, 231 (1): 77–81. PMID 9070223. doi:10.1006/bbrc.1997.6004.
- ^ Yan HX, He YQ, Dong H, Zhang P, Zeng JZ, Cao HF, et al. Physical and functional interaction between receptor-like protein tyrosine phosphatase PCP-2 and beta-catenin. Biochemistry. December 2002, 41 (52): 15854–15860. PMID 12501215. doi:10.1021/bi026095u.
- ^ He Y, Yan H, Dong H, Zhang P, Tang L, Qiu X, et al. Structural basis of interaction between protein tyrosine phosphatase PCP-2 and beta-catenin. Science in China Series C: Life Sciences. April 2005, 48 (2): 163–167. PMID 15986889. S2CID 20799629. doi:10.1007/bf02879669.
- ^ Tesco G, Kim TW, Diehlmann A, Beyreuther K, Tanzi RE. Abrogation of the presenilin 1/beta-catenin interaction and preservation of the heterodimeric presenilin 1 complex following caspase activation. The Journal of Biological Chemistry. December 1998, 273 (51): 33909–33914. PMID 9852041. doi:10.1074/jbc.273.51.33909
 .
.
- ^ Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, et al. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. The Journal of Neuroscience. June 1999, 19 (11): 4229–4237. PMC 6782616
 . PMID 10341227. doi:10.1523/JNEUROSCI.19-11-04229.1999.
. PMID 10341227. doi:10.1523/JNEUROSCI.19-11-04229.1999.
- ^ Murayama M, Tanaka S, Palacino J, Murayama O, Honda T, Sun X, et al. Direct association of presenilin-1 with beta-catenin. FEBS Letters. August 1998, 433 (1–2): 73–77. PMID 9738936. S2CID 85416623. doi:10.1016/S0014-5793(98)00886-2.
- ^ Puppo F, Thomé V, Lhoumeau AC, Cibois M, Gangar A, Lembo F, et al. Protein tyrosine kinase 7 has a conserved role in Wnt/β-catenin canonical signalling. EMBO Reports. January 2011, 12 (1): 43–49. PMC 3024124
 . PMID 21132015. doi:10.1038/embor.2010.185.
. PMID 21132015. doi:10.1038/embor.2010.185.
- ^ Bauer A, Huber O, Kemler R. Pontin52, an interaction partner of beta-catenin, binds to the TATA box binding protein. Proceedings of the National Academy of Sciences of the United States of America. December 1998, 95 (25): 14787–14792. Bibcode:1998PNAS...9514787B. PMC 24527
 . PMID 9843967. doi:10.1073/pnas.95.25.14787
. PMID 9843967. doi:10.1073/pnas.95.25.14787  .
.
- ^ Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. The EMBO Journal. September 2001, 20 (17): 4935–4943. PMC 125268
 . PMID 11532957. doi:10.1093/emboj/20.17.4935.
. PMID 11532957. doi:10.1093/emboj/20.17.4935.
- ^ Taya S, Yamamoto T, Kanai-Azuma M, Wood SA, Kaibuchi K. The deubiquitinating enzyme Fam interacts with and stabilizes beta-catenin. Genes to Cells. December 1999, 4 (12): 757–767. PMID 10620020. S2CID 85747886. doi:10.1046/j.1365-2443.1999.00297.x.
- ^ Sinn HW, Balsamo J, Lilien J, Lin JJ. Localization of the novel Xin protein to the adherens junction complex in cardiac and skeletal muscle during development. Developmental Dynamics. September 2002, 225 (1): 1–13. PMID 12203715. S2CID 23393425. doi:10.1002/dvdy.10131
 .
.
- ^ Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking beta-catenin to androgen-signaling pathway. The Journal of Biological Chemistry. March 2002, 277 (13): 11336–11344. PMID 11792709. doi:10.1074/jbc.M111962200
 .
.
- ^ Masiello D, Chen SY, Xu Y, Verhoeven MC, Choi E, Hollenberg AN, Balk SP. Recruitment of beta-catenin by wild-type or mutant androgen receptors correlates with ligand-stimulated growth of prostate cancer cells. Molecular Endocrinology. October 2004, 18 (10): 2388–2401. PMID 15256534. doi:10.1210/me.2003-0436
 .
.
- ^ Song LN, Coghlan M, Gelmann EP. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Molecular Endocrinology. January 2004, 18 (1): 70–85. PMID 14593076. doi:10.1210/me.2003-0189
 .
.
- ^ Amir AL, Barua M, McKnight NC, Cheng S, Yuan X, Balk SP. A direct beta-catenin-independent interaction between androgen receptor and T cell factor 4. The Journal of Biological Chemistry. August 2003, 278 (33): 30828–30834. PMID 12799378. doi:10.1074/jbc.M301208200
 .
.
- ^ Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, Wierman ME. Liganded androgen receptor interaction with beta-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. The Journal of Biological Chemistry. June 2002, 277 (23): 20702–20710. PMID 11916967. doi:10.1074/jbc.M200545200
 .
.
- ^ Cartegni L, di Barletta MR, Barresi R, Squarzoni S, Sabatelli P, Maraldi N, et al. Heart-specific localization of emerin: new insights into Emery-Dreifuss muscular dystrophy. Human Molecular Genetics. December 1997, 6 (13): 2257–2264. PMID 9361031. doi:10.1093/hmg/6.13.2257
 .
.
- ^ Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. The EMBO Journal. July 2006, 25 (14): 3275–3285. PMC 1523183
 . PMID 16858403. doi:10.1038/sj.emboj.7601230.
. PMID 16858403. doi:10.1038/sj.emboj.7601230.