烏帕替尼
外觀
此條目可參照英語維基百科相應條目來擴充。 (2024年11月5日) |
 | |
| 臨床資料 | |
|---|---|
| 讀音 | /juˌpædəˈsaɪtɪnɪb/ ew-PAD-ə-SY-ti-nib |
| 商品名 | Rinvoq |
| 其他名稱 | ABT-494 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619051 |
| 核准狀況 |
|
| 懷孕分級 | |
| 給藥途徑 | 口服給藥 |
| 藥物類別 | Janus激酶抑制劑 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 血漿蛋白結合率 | 52% |
| 藥物代謝 | 肝臟 (CYP3A 主, CYP2D6 輔)[12] |
| 代謝產物 | M4, 酰基 葡萄糖苷酸 |
| 生物半衰期 | 9–14[11] (6–15[12]) 小時 |
| 排泄途徑 | 基本不變,糞便(38%)和尿液(24%)[11] |
| 識別資訊 | |
| |
| CAS號 | 1310726-60-3 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化學資訊 | |
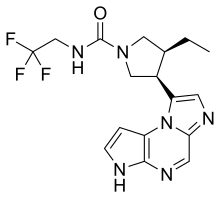
| 化學式 | C17H19F3N6O |
| 摩爾質量 | 380.38 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
烏帕替尼(商品名:英語:Rinvoq,台灣:銳虎) ,類風濕性關節炎、乾癬性關節炎、異位性皮膚炎、潰瘍性結腸炎、克隆氏症、僵直性脊椎炎和中軸型脊柱關節炎[9] [10]。因療效與注射類型的蛋白質大分子生物製劑相近,在台灣又通稱為口服小分子標靶藥物[13]。 標靶目標是JAK激酶,它與引發炎的過程有關,藥物能阻斷它的作用,進而控制身體的發炎反應[10]。因此稱為Janus激酶抑制劑[9] [14] [10]。
常見副作用包括上呼吸道感染(如感冒、鼻竇炎)、噁心、咳嗽和發燒[15] [16]。
烏帕替尼在2019年獲得歐洲和美國的醫療使用許可[15] [16]。
參考文獻
[編輯]- ^ Upadacitinib (Rinvoq) Use During Pregnancy. Drugs.com. 23 September 2019 [17 March 2020]. (原始內容存檔於18 March 2020).
- ^ AusPAR: Upadacitinib. Therapeutic Goods Administration (TGA). 25 August 2021 [4 September 2021]. (原始內容存檔於4 September 2021).
- ^ Rinvoq (Abbvie Pty Ltd). [9 November 2022]. (原始內容存檔於9 November 2022).
- ^ Rinvoq (Abbvie Pty Ltd). Therapeutic Goods Administration (TGA). 16 February 2023 [9 April 2023]. (原始內容存檔於18 March 2023).
- ^ Rinvoq Product information. Health Canada. 25 April 2012 [29 May 2022]. (原始內容存檔於30 May 2022).
- ^ Summary Basis of Decision (SBD) for Rinvoq. Health Canada. 23 October 2014 [29 May 2022]. (原始內容存檔於31 May 2022).
- ^ Regulatory Decision Summary for Rinvoq. Drug and Health Products Portal. 21 July 2023 [1 April 2024].
- ^ Rinvoq 15 mg prolonged-release tablets - Summary of Product Characteristics (SmPC). (emc). 1 March 2020 [22 August 2020]. (原始內容存檔於27 August 2021).
- ^ 9.0 9.1 9.2 Rinvoq- upadacitinib tablet, extended release. DailyMed. 1 March 2020 [29 April 2020]. (原始內容存檔於27 August 2021).
- ^ 10.0 10.1 10.2 10.3 Rinvoq EPAR. European Medicines Agency (EMA). 16 October 2019 [29 April 2020]. (原始內容存檔於20 October 2020).
- ^ 11.0 11.1 Rinvoq: EPAR – Public assessment report (PDF). European Medicines Agency. 5 March 2020 [21 July 2020]. (原始內容存檔 (PDF)於21 July 2020).
- ^ 12.0 12.1 Mohamed MF, Camp HS, Jiang P, Padley RJ, Asatryan A, Othman AA. Pharmacokinetics, Safety and Tolerability of ABT-494, a Novel Selective JAK 1 Inhibitor, in Healthy Volunteers and Participants with Rheumatoid Arthritis. Clinical Pharmacokinetics. December 2016, 55 (12): 1547–1558. PMID 27272171. S2CID 39036534. doi:10.1007/s40262-016-0419-y.
- ^ 類風濕性關節炎 - 類風濕性關節炎的藥物與治療. 台灣免疫風濕疾病關懷協會. 2017-01-20 (中文(臺灣)).
- ^ Drug Trials Snapshots: Rinvoq. U.S. Food and Drug Administration (FDA). 16 August 2019 [18 March 2020]. (原始內容存檔於5 August 2020).
- ^ 15.0 15.1 Rinvoq. [13 September 2021]. (原始內容存檔於20 October 2020).
- ^ 16.0 16.1 Upadacitinib Monograph for Professionals. Drugs.com. [13 September 2021]. (原始內容存檔於22 August 2021) (英語).
