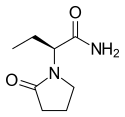
左乙拉西坦
外觀
| |||
| 臨床資料 | |||
|---|---|---|---|
| 讀音 | /lɛvɪtɪˈræsɪtæm/ | ||
| 商品名 | Keppra, Elepsia, Spritam, others | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a699059 | ||
| 核准狀況 | |||
| 懷孕分級 | |||
| 給藥途徑 | 口服給藥、靜脈注射 | ||
| 藥物類別 | 消旋坦 抗癲癎藥物 | ||
| ATC碼 | |||
| 法律規範狀態 | |||
| 法律規範 |
| ||
| 藥物動力學數據 | |||
| 生物利用度 | ≈100% | ||
| 血漿蛋白結合率 | <10% | ||
| 藥物代謝 | 乙醯胺基團的酵素水解 | ||
| 生物半衰期 | 6–8小時 | ||
| 排泄途徑 | 腎 | ||
| 識別資訊 | |||
| |||
| CAS號 | 102767-28-2 | ||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.121.571 | ||
| 化學資訊 | |||
| 化學式 | C8H14N2O2 | ||
| 摩爾質量 | 170.21 g·mol−1 | ||
| 3D模型(JSmol) | |||
| |||
| |||
左乙拉西坦(Levetiracetam)是一種治療癲癇的藥物[7]。適用的治療包括:部分性發作、肌陣攣性發作或強直-陣攣性發作[7]。有口服(即時釋放或緩慢釋放的修飾釋放劑型),或靜脈注射[7]。
常見的副作用包括嗜睡、頭暈、疲倦和攻擊性[7]。嚴重的副作用可能包括精神病、自殺和過敏,如史蒂芬斯-強森症候群和過敏性休克[7]。懷孕期使用的安全性仍不清楚,但哺乳期使用似乎安全[8]。它是乙拉西坦的左旋-對映異構體[9]。詳細的作用機制尚不清楚[7]。
此藥於1999年在美國取得醫療使用許可,[7]並名列世界衛生組織的基本藥物清單[10]。已有學名藥流通於市[11]。 2020年,它在美國最常用的處方藥中名列第92位,處方量超過700萬張[12] [13]。
參考文獻
[編輯]- ^ Levetiracetam Use During Pregnancy. Drugs.com. [5 March 2019]. (原始內容存檔於6 March 2019).
- ^ Keppra 100 mg/ml concentrate for solution for infusion - Summary of Product Characteristics (SmPC). (emc). [9 September 2020]. (原始內容存檔於24 October 2021).
- ^ Keppra- levetiracetam tablet, film coated Keppra- levetiracetam solution. DailyMed. 5 November 2019 [9 September 2020]. (原始內容存檔於7 August 2020).
- ^ Keppra XR- levetiracetam tablet, film coated, extended release. DailyMed. 4 November 2019 [9 September 2020]. (原始內容存檔於29 July 2021).
- ^ Keppra- levetiracetam injection, solution, concentrate. DailyMed. 4 November 2019 [9 September 2020]. (原始內容存檔於21 January 2016).
- ^ Anvisa. RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control]. Diário Oficial da União. 31 March 2023 (4 April 2023) [16 August 2023]. (原始內容存檔於3 August 2023) (巴西葡萄牙語).
- ^ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Levetiracetam Monograph for Professionals. Drugs.com. AHFS. [14 January 2019]. (原始內容存檔於24 March 2019) (英語).
- ^ Levetiracetam Use During Pregnancy. Drugs.com. [5 March 2019]. (原始內容存檔於6 March 2019) (英語).
- ^ Cavanna, Andrea E. Behavioural Neurology of Anti-Epileptic Drugs: A Practical Guide. Oxford University Press. 2018: 17 [5 March 2019]. ISBN 9780198791577. (原始內容存檔於6 March 2019) (英語).
- ^ World Health Organization. The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ^ British national formulary: BNF 76 76. Pharmaceutical Press. 2018: 319. ISBN 9780857113382.
- ^ The Top 300 of 2020. ClinCalc. [7 October 2022]. (原始內容存檔於12 February 2021).
- ^ Levetiracetam - Drug Usage Statistics. ClinCalc. [7 October 2022]. (原始內容存檔於28 February 2020).